Class 11 Chemistry Periodic classification
CLASSIFICATION OF ELEMENTS
# History :
1) Dobereiner's Triads : He arranged the known elements in increasing order of the atomic mass , so that the atomic mass of middle element is Avg. Sum of other 2 elements
1st Triad : Li (7) Na (23) K (35)
2nd Triad : Ca (40) Sr (88) Ba (137)
3rd Triad : Cl(35.5) Br (80) I (127)
2) Jelluric Helix :
- He Arrange all the known elements in increasing order of atomic mass .
- He Formed cylindrical form of periodic table ,
3) Newland's Law of Octaves :
- He arranged the element in increasing order of atomic mass such that the property of every eighth element is same as the first element .
Li Be B C N O F
Na Mg Al Si P S Cl
K Ca Sc
- This was only Applicable Till Ca .
4) Lother Meyer & Mendelev :
A) Lothar meyer : He drew the graph of atomic volume V/S Atomic weight of all the know element
* The descending portion of the curve is occupied by less electropositive elements such as Be , Mg , Ca Sr , Ba .
* The ascending portion of the curve was occupied by most electronegative element such as F , Cl , Br, I
* Lothar mayer arranged all the known element in increasing order of atomic weight & formed the periodic table .
B) D.Mendeleev : He also arranged all the known element in increasing order of atomic weight and he made sure that the elements coming in same group must have same properties .
# Mendeleev's Periodic Law :
- The properties of element are periodic Function of their Atomic weight .
☆ At.wt of Te > At.wt of I
O F
S Cl
Se Br
Te I
5) Modern Periodic Table :
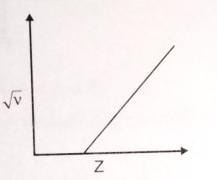
☆ Henry Moseley : Idea was given or Formed by .
√V = a ( z - b ) v = frequency of x-ray
z = Atomic No .
a, b = Henrey Constant
☆ He drew the graph of √V V/S At.wt & √V v/s At.no
Modern Periodic Law :
☆ The properties of elements are periodic function of their atomic number .
☆ Modern Periodic Table : 7Rows , 7Periods
18Colums , 18Group
☆☆ Group 1& 2 : S - Block element (Ns1-2)
Group 1 - Alkali Metals (Ns1)
☆☆ Group 13 - 18 : p-block elements (Ns2 np1-6)
Group 18 - Zeroth group or Noble Gas group
Group 17 - Halogens or salt forming elements
Group 16 - Chalogens or Ore forming elements
Group 15 - Nictrogens ir Poisonous Elemets
* They are also known as Transition Metals
* Zn , Cd , Hg - Pseudotrasition Metals
* Grp 3 period 6 : Lanthanoids
* Grp 3 period 7 : Actinoids
# Nomenclature of elements of atomic no > 100
0 : nil
1: un
2: bi
3: tri
4: quad
5: pent
6: hex
7: sept
8: oct
9: enn
☆☆ Number should be ended with " ium "
☆☆ Remember The actual/official names of elements from 101 to 110
# Periodic Trends :
1) Atomic Size : It is measured in Terms of atomic radius which means the distance b/w nucleus and the outer most orbit of the atom .
There are four types of atomic radius which are measured :
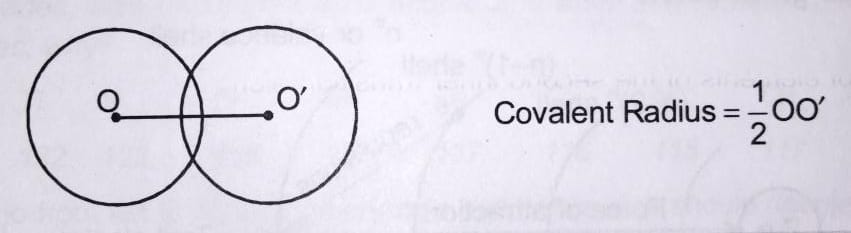
i) COVALENT RADIUS : Covalent Radius = X/2
-- Half of the distance b/w the nucleus of two same atom bonded by single covalent bond .
ii) Vanderwaal's Radius : Specially of nobel gases & non bonded atom
-- Vanderwaal's Radius = y/2
y= min. Dist. b/w 2 non- bonded atom
- Measured Mostly for noble gases
iii) METALLIC RADIUS : Homoatomic
-- Metallic Radius = Z/2
-- Z = metallic bond length in the crystal lattice of metal
☆☆ Vanderwaal's Radius > Metallic Radius > Covalent Radius
iv) IONIC RADIUS :
Cationic Radius
Na → Na+ e-
Anionic Radius
Cl + e- → cl-
Radius of parents atom > Radius of cation
:::::::: Size ∝ 1 / Zeff
SHEILDING EFFECT OR SCREENING EFFECTS :
- The inner e- & the e- in the same orbit prevents the nuclear force from the going outwards.
- This is called as sheilding effect and the nuclear force experienced by the outermost e- due to sheilding effect is called as effectivie nuclear charge Zeff .
- The decreasing sheilding power of sub - shells is as follows :
(most powerfull ) S > p > d > f
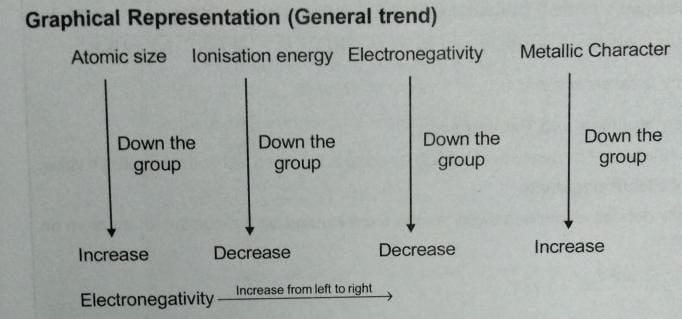
1) PERIODIC TRENDS OF ATOMIC SIZE :
i) While moving down the group every time the e- is added to new shell , the Zeff of the outermost e- decreases and hence while moving down the group atomic size increase .
ii) While moving across the period , shielding effect remain almost constant but as atomic number increase , zeff also increases
* Hence , while moving across the period atomic size decrease
# EXCEPTION :
In grp 13 :
B , Al , Ga ,In , Tl
B < Al > Ga < In > Tl
Due to poor shielding of d- orbital = Zeff increases
ISONISATION ENTHALPY OR ISONIZATION POTENTIAL :
- Amt. of energy required to remove an e- from outermost orbit of an isolated gaseous atom
Na(s) → Na+ + e- I.E ✕
Na(g) → Na+ + e- I.E ✓
ELECTRON GAIN ENTHALPY :
It is the enthalpy change when an electron is added to the isolated gaseous neutral atom. Electron gain enthalpy provides a measure of the ease with which an atom adds an electron to form anion.
Depending on the element, enthalpy change in process may be endothermic or exothermic When an electron is added to gaseous neutral atom, energy is released also known as electron affinity. (electron gain enthalpy is negative). If energy is absorbed, electron gain enthalpy is positive.
Factors affecting the Electron gain enthalpy :
(1) Atomic size: Electron gain enthalpy is inversely proportional to the atomic size. Generally smaller the size higher will be the electron gain enthalpy
(ii) Effective nuclear charge: Higher the effective nuclear charge, higher will be negative electron gain enthalpy.
(iii) Screening effect: Higher the screening effect, lower will be effective nuclear charge and hence lower will be negative electron gain enthalpy
(iv) Electronic configuration: Half filled and fully filled electronic configuration is extra stable, so addition of an electron to that system is difficult and electron gain enthalpy is zero or positive.
ELECTRONEGATIVITY :
Electronegativity is a measure of the tendency of an element to attract bonded electron pair towards itself in a covalently bonded molecule.
Factors on which electronegativity depends:
(1) Atomic size: Electronegativity is inversely proportional to the size.
(ii) Effective Nuclear Charge: Electronegativity is directly proportional to the effective nuclear charge.